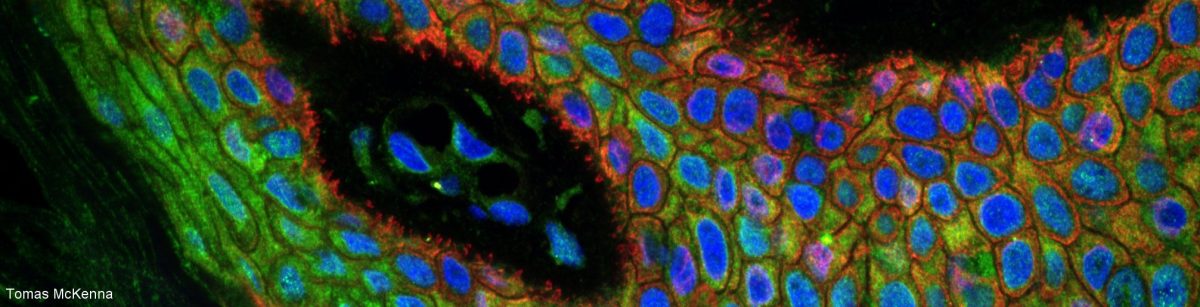
Together with the coverslip and the immersion medium (oil, water, glycerol or air), the sample mounting medium is part of the design of a microscopy objective. Matching the refraction index of the sample to the one recommended by the manufacturer of the objective will make the sample transparent for the objective, drastically improving fluorescence microscopy in samples thicker than a couple of um (i.e. anything except fluorescent beads!).
Not matching the refraction indices is equivalent to watching something through a wet window… Far from optimal! :-/
The refraction index recommended by the manufacturer is the same as the RI of the immersion medium: 1.52 for an oil immersion objective, 1.47 for a glycerol objective, 1.33 for a water objective, 1 for an air objective.
This article compares 7 mounting media and their effect on the refraction index of brain samples. CFM3 seems to be a cool mounting medium. The company that produces it has partially paid for the study but it sounds worth a try anyway!
In the same vein, this article presents a non-toxic way to change the refraction index of cell culture medium (not the sample) to improve imaging of live samples. Sounds pretty promising to grow live organoids which quickly become opaque. This will also be very useful when clearing samples as the sample chamber on a light sheet microscope is big so this is a cheap way to fill the chamber for imaging. 🙂
If you try any of these 2 chemicals, please leave a comment to let us know how it went! 🙂
Like this:
Like Loading...