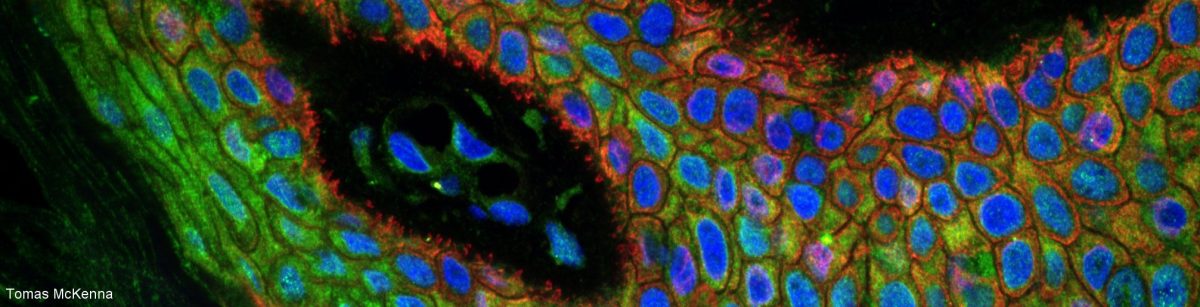
As you all (nearlyish) know, one should never place a sample on a thick glass slide and add a coverslip. Instead, the sample should be placed on the coverslip then covered with a thick glass slide. And the coverslip should be 170 um thick (also labelled thickness #1.5).
Why is that? Because the coverslip is part of the design of the objective and all objectives from all manufacturers are designed to image through 170 um glass and assuming that the sample is directly in contact with the coverslip.
What about superfrost slides that one uses to make sure tissue sections don’t float away during antigen retrieval? No worry! You can make your own superfrost coverslips. It is cheap and you can prepare tons at the same time. Here is the protocol (and pasted below).
Not convinced? You will only see the difference when you compare side by side! The images of your tissue will be much sharper if the sample is on the coverslip because when you put your sample on the slide, either the thick glass or the mounting medium end up between the objective and the sample. The objective is not designed for this. ?
Here is the protocol:
Reagents Required
- Gelatin-coating solution: 1 L deionized H2O, 5 g gelatin, 0.5 g chromium potassium sulfate dodecahydrate CrK(SO4)2 · 12H2O
Materials
- Filter units
- Histological slides
- Hot plate with magnetic stirrer
- Slide racks
- Staining dish
- Thermometer
Procedure
- Prepare the gelatin-coating solution by dissolving 5 g of gelatin in 1 L of heated, deionized H2O (temperature should not exceed 45 °C).
- After the gelatin has dissolved, add 0.5 g of chromium potassium sulfate dodecahydrate. Chromium potassium sulfate dodecahydrate will positively charge the slides allowing them to attract negatively charged tissue sections.
- Filter this solution and store at 2-8 °C until use. It is recommended that this solution be filtered again immediately before use (adjust to room temperature before filtration).
- Place the histological slides into metal racks.
Note: The slides should be cleaned by washing them in soapy water and rinsing them thoroughly, first in tap water and finally in deionized water. - Dip the racks containing the slides 3 to 5 times (~5 seconds each) into the gelatin-coating solution.
- Remove the racks containing the slides and let them drain. Blot excess solution from the racks onto filter paper (gently tap the racks against the filter paper for better drainage).
- Place the racks containing the slides on the lab bench and cover them with paper towels to protect them from dust.
- Dry at room temperature for 48 hours.
- Dried slides can be put back into the boxes that they arrived in and stored at room temperature until use. Slides intended for cryostat sections can be stored at -20 °C.